News

Lausanne one, virus nil
Francesco Stellacci, professor at the École polytechnique fédérale de Lausanne, envisions a two-pronged approach to treating viral infections of any kind: should a virus manage to evade his novel broad-spectrum drug, a tailor-made antiviral will step in to finish the job. Thanks to the Werner Siemens Foundation, Stellacci can now set up a new research centre to realise his innovative strategy.
The coronavirus pandemic has changed our lives—a statement that rings doubly true for Francesco Stellacci, professor of materials science at the École polytechnique fédérale de Lausanne (EPFL). Like the rest of us, he has been forced to come to terms with the pandemic as a private individual. But the situation has also had a major impact on his professional life. Indeed, before the pandemic, people often looked somewhat askance when Stellacci talked about his research focus of the past ten years: developing a broad-based antiviral drug. Is that even possible, they wanted to know. And aren’t vaccines more important?
Since the advent of the novel coronavirus, however, Stellacci’s idea of a broad-based antiviral drug has gained significant traction. To be sure, the international research community attained unprecedented success in developing vaccines against Sars-CoV-2, and that in record time. Nevertheless, it remains unclear whether the vaccines will offer protection against all new variants of the coronavirus.

Broad-spectrum antiviral drugs
Also far from certain is whether the research community will be able to produce a vaccine as quickly during the next pandemic. “That’s why it’s important that we also seek drugs that are effective against viruses in addition to developing vaccines,” says Stellacci. An antiviral that can treat a wide range of virus infections would not only save human lives: it would also preserve the world from reliving the chaos of the past year should another pandemic arise.
Thanks to funding from the Werner Siemens Foundation, Stellacci and his team at the EPFL Supramolecular Nanomaterials and Interfaces Laboratory can now dedicate their energies to developing a broad-spectrum antiviral drug. Their collaboration partners include specialists in virology and infectious diseases at the University of Geneva and the Geneva University Hospitals.
Lausanne takes the lead
To create their drug, the researchers take specific sugar molecules and modify them so that they can outsmart a virus. The method is as follows: the altered molecules pretend to be parts of a human cell, whereupon the virus is tricked into bonding with these “dummy” cells—a fatal error, because the sugar molecules are equipped with “tentacles” that exert so much pressure on the virus that it explodes. Unlike existing antiviral drugs, this approach both inhibits reproduction and irreversibly destroys the virus. The score is now Lausanne one, virus nil.
Since the spring of 2020, Stellacci and his team have developed two versions of a broad-spectrum antiviral agent, as the researchers need two different types of sugar molecules to function as the dummy cells in order to lure as many different virus types as possible into latching on to the molecules, where they are squeezed until they burst. The first agent is designed to treat Covid-19, Zika, Dengue fever and other viral diseases. The second agent specifically aims to combat the many different kinds of influenza viruses.
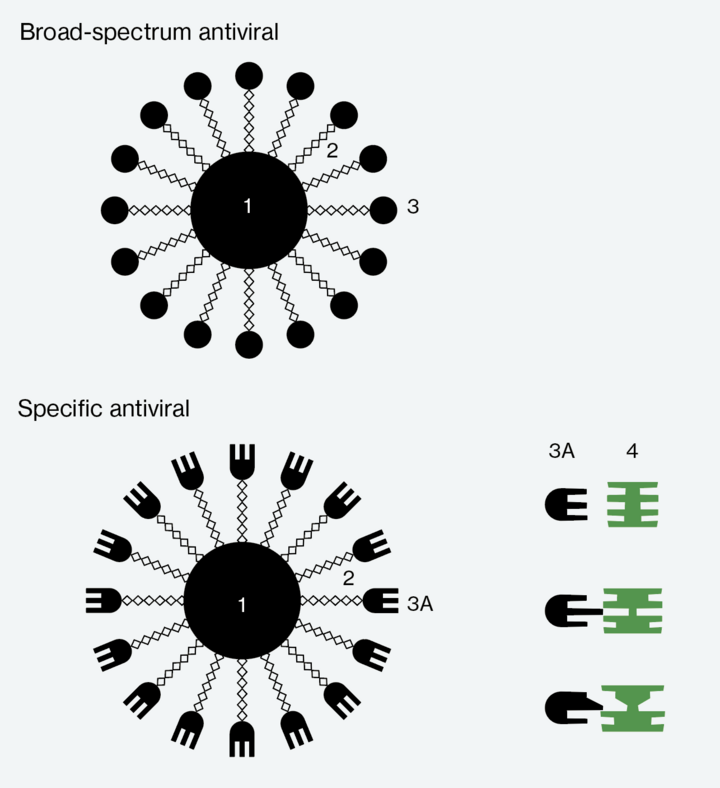
2 Tentacles
3 Universal docking sites
3A Specific docking sites
4 Docking sites on the virus

Initial test results are encouraging
Both agents were already so far advanced in 2020 that initial studies on animals would have been possible. However, the experiments were delayed, because the external project partners were busy conducting tests for Covid-19 vaccines. The trials finally began in May 2021, and the novel agents were tested for both safety and efficacy.
The initial results are promising. The agent designed to treat influenza has a significant advantage over Tamiflu, the most effective flu drug developed to date. Tamiflu is effective only when taken within thirty-six hours of being infected, but the first flu symptoms generally present roughly twenty-four hours after infection. “By the time a patient seeks medical help and receives Tamiflu, it’s often already too late,” Stellacci says. The agent developed by his team remains effective even later in the course of a disease, making it much more suitable for medical care.
The agent has also been tested against Sars-CoV-2 and related viruses. Its toxicology levels were safe in initial tests on animals, and its efficacy results very encouraging. The aim is to have completed extensive tests of both broad-spectrum agents in animal models by 2023 so that clinical trials can begin.
A specific antiviral
After awarding Stellacci a one-year grant in 2020, the Werner Siemens Foundation decided to provide long-term funding for his innovative project. This financial support is what made it possible for Stellacci in 2021 to turn his attention to realising another of his visionary projects. To protect humans (as well as animals and plants) from viral diseases we need more than broad-spectrum antiviral drugs. The situation is similar to bacterial diseases, as Stellacci explains: “Even the best broad-spectrum antibiotic isn’t effective against every kind of bacteria.” Serious bacterial infections are first treated with a broad-spectrum antibiotic, generally penicillin, and patients often require no further treatment. But if they fail to recover, the exact pathogen is identified in the lab, and patients receive an antibiotic that is effective against this specific strain of bacteria.
Stellacci envisions a similar approach to treating viral diseases: first the patient should be given a broad-spectrum antiviral. If this fails to bring about the desired results, the virus type is identified in the lab and the patient then receives a specific antiviral drug.
To develop specific antiviral drugs, the researchers are aiming to create a standardised procedure that will enable them to produce effective agents for both already known viruses and emerging viruses within a short time span. The coronavirus pandemic has placed this need in sharp relief: although vaccines were developed very quickly, finding drugs that are effective in treating Covid-19 has proven much more elusive.
![[Translate to English:] Double trouble for viruses—How Professor Stellacci’s project will help fight future pandemics [Translate to English:] Illustration of how Professor Stellacci’s project will help fight future pandemics.](/fileadmin/_processed_/0/e/csm_08_Antivirus_EN_736fa4d6ad.png)
Learning from the body’s own defence mechanisms
To realise his vision of a specific antiviral, Francesco Stellacci plans to combine two approaches. The first is his broad-spectrum antiviral drug that applies mechanical pressure to destroy viruses. The second concerns developing a specific antiviral drug based on the most effective way to combat viruses that evolution has come up with: antibodies. The great advantage of antibodies is that they have the right shape to bind to specific parts of a virus, akin to how the right key fits into a lock. Once antibodies have latched on to a virus, its binding sites are occupied and it can no longer infect human cells.
Antibodies for every kind of virus can be taken from the blood of people who have recovered from a virus—and we are lucky to have antibodies for all viral infections, as the human race has so far survived every disease caused by a virus. For instance, the plasma of recuperated Covid-19 patients was used to develop Sars-CoV-2 vaccines. Francesco Stellacci is convinced that antiviral drugs can be built using the same principle: “We’ve rarely taken advantage of this possibility, and there are currently only very few antibody-based drugs for treating viral infections.”
Tentacles combined with antibodies
Like the broad-spectrum antivirals, Stellacci’s specific antiviral drugs will consist of modified sugar molecules and tentacles. The difference is that they will also contain a part of the antibody that binds to the specific virus (see diagram page 50). This is how the researchers can exploit the advantages of antibodies while also avoiding their drawbacks, the greatest of which being that antibodies block viruses rather than destroying them. In Stellacci’s model, the sugar molecule and its tentacles are responsible for the task of irreversibly damaging the virus. The result will be an agent that combines the antibody’s precise binding to a virus with the capacity to destroy the pathogens. That would make the score Lausanne two, virus nil.
To prevent the viruses from becoming resistant to an agent (as is increasingly the case with antibiotic drugs) the specific antivirals are designed to target what is known as the “conserved parts” of a virus. These parts of a virus rarely mutate, as they are so essential to its survival. Once an agent binds to this critical site, the virus cannot easily free itself by mutating, thus increasing the probability that the agent will retain its efficacy for a long time.
A new research centre
Stellacci plans to use the long-term funding from the Werner Siemens Foundation to set up a centre of excellence at EPFL: The Werner Siemens Foundation Center for Antivirals Research. The centre’s ambitious goal is to ensure that an agent to treat a new viral disease is ready for clinical trials just six months after the virus’s genome has been sequenced. A standardised method for creating different specific agents is what will make this possible. The approach will also facilitate an efficient supply of antiviral agents for patients in Switzerland and Europe.
Francesco Stellacci and his team now have until 2023 to prove their concept. By then, they plan to have tested not only the broad-spectrum agents but also the first specific antiviral agents in animal models. In addition, they will submit a detailed strategy for establishing the Werner Siemens Foundation Center for Antivirals Research.
Text: Adrian Ritter
Photos: Kellenberger Kaminski Photographie/ETH Board
Illustration: bigfish AG