Analysing gaits and simulating fractures
The smart implants project is entering its second phase: after gaining a good overall understanding of how broken bones heal, the researchers are now seeking ways to consolidate this knowledge—and build a novel implant.
Three years of preparatory research were needed before the team could really begin making inroads, says Tim Pohlemann, head of the Department for Trauma, Hand and Reconstructive Surgery at the Saarland University Medical Center. He and his colleague Bergita Ganse lead the smart implants project, which has received funding from the Werner Siemens Foundation since 2019. In the project, the Saarland researchers apply novel technologies from the fields of materials science and engineering to design intelligent implants that promote healing in complex bone fractures. The devices will be able to carry out three different tasks, virtually at the same time: moni-tor the fracture site, detect incorrect weight-bearing—and stimulate healing through targeted, autonomous movements at the site of a break.
Pohlemann explains that all the groups involved in the project—the surgery unit, the simulation and computer technology teams as well as the technical developers—first had to make advances in their fields. “But now we’re ready to consolidate our new knowledge and to integrate it into a functioning implant,” he says.
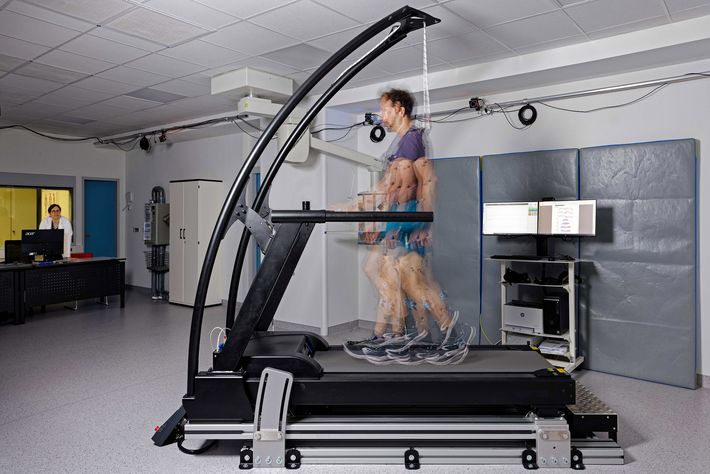
Was it the patient or a bus?
Gait analysis is one area where the researchers made particularly good progress last year. “We’ve now been able to gather data on how bone fractures healed in a total of ninety patients—in studies conducted here in the lab and at the patients’ homes,” says Bergita Ganse, Werner Siemens Foundation Endowed Chair for innovative implant development at Saarland University. For this, the patients had so-called wearables installed in their shoes: thin insoles equipped with sensors that measure pressure, acceleration and weight-bearing factors.
“Using the insoles, we generated the first long-term data as well as many other findings that have now been published,” Ganse says. Understanding which parameters change during healing—and how they change—is of particular importance in the project, as is using this information to identify those values that quickly indicate suboptimal healing. “Many patients recover very well,” Ganse explains, “but unfortunately there are still too many cases that don’t progress as they should.” She adds that the difference in strength between a person’s right and left foot could be an indicator of poor healing. Another might be the movement of the centre of gravity on the sole of the foot. “The data also show us how often a patient puts up their leg to rest,” Ganse says. “Now, the next step is analysing each individual pattern of motion and its distribution in connection with the healing prognosis.”
Another critical aspect in the project is understanding why pressure and acceleration measurements change. “When the insoles deliver data saying there was an uptake in speed, we need to know whether the patient was running or sitting on a bus,” Pohlemann says. The researchers have also studied the type of surface (asphalt, sand, grass, gravel) as well as topography (walking uphill or downhill, climbing stairs) to ascertain what effects they have on the force curves. Patient safety is the reason for needing this information, as Bergita Ganse explains: if a problem arises, the smart implant embedded in the leg of a patient should emit a warning and, if necessary, counteract incorrect weight-bearing. In order to do this, the implant must be capable of recognising the myriad factors involved in these types of changes, but standard statistical methods are often not able to make the necessary calculations. For this reason, the researchers have developed targeted AI algorithms to detect correlations that are otherwise difficult to identify.
How much movement is enough?
The researchers have also attained excellent results in their computer simulations. “We used data from computer tomography scans of patients to simulate fractures at different places and angles,” Ganse says. Here, the goal is to find out how much the implant should move—depending on the type of fracture—to best steer healing.
It’s no easy task. One problem arises in the case of complex fractures such as wedge-shaped breaks that make pressure and weight-bearing distribution difficult to calculate. Another challenge lies in considering changes that occur over time. “Because tissues become stiffer as they heal, we need other forces to act on the fracture site,” Bergita Ganse explains.
The team’s computer simulations are a key step towards understanding what an implant must be able to do, Tim Pohlemann adds: “This is at the very heart of our project: we want to build the implant so that, in the end, it’s capable of triggering movements and changes in weight-bearing that support healing effectively.”




